DNA Hormone Health Test
We offer two types of tests; Lab Tests and Rapid Tests. This product is under the category Lab Tests. See all our Lab Tests by following the link.
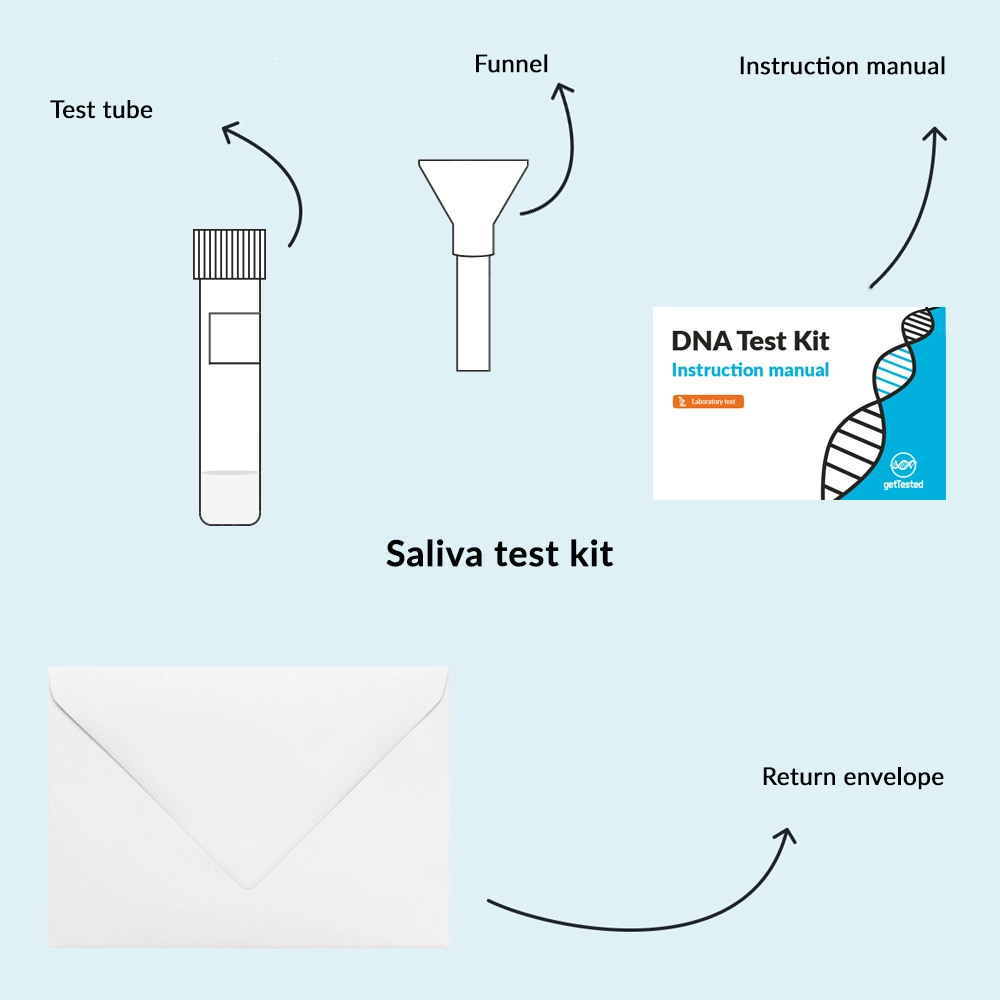
See allWe offer several different options of testing methods. This test is done with Saliva. See all tests done with Saliva by following the link.
See allGetTested’s DNA Hormone Health test analyses specific genes to assess hormone health, including T3, T4, Free T4, TSH, Testosterone, and FSH. This targeted approach helps identify genetic factors influencing hormone production and metabolism. Ideal for individuals experiencing hormone-related symptoms or those interested in personalized health insights, the test simplifies understanding your hormone profile through a straightforward saliva sample process. Results provide actionable recommendations, delivered with confidentiality and privacy assurance.
The price covers the return shipping. You’ll receive your detailed results digitally within 6-8 weeks.
Save more by bundling DNA tests. Order multiple tests together for better value from our wide range.
Save more by bundling DNA tests. Order multiple tests together for better value from our wide range.
- In stock
- At-home test
- Results 6-8 weeks

Get 5% off on 2 Lab tests, and 10% off on 3 Lab tests or more.
What is analyzed in the test?
T3 (Triiodothyronine)
T4 (Thyroxine)
Free T4
TSH
Testosterone
Bioavailable Testosterone
FSH
Ghrelin
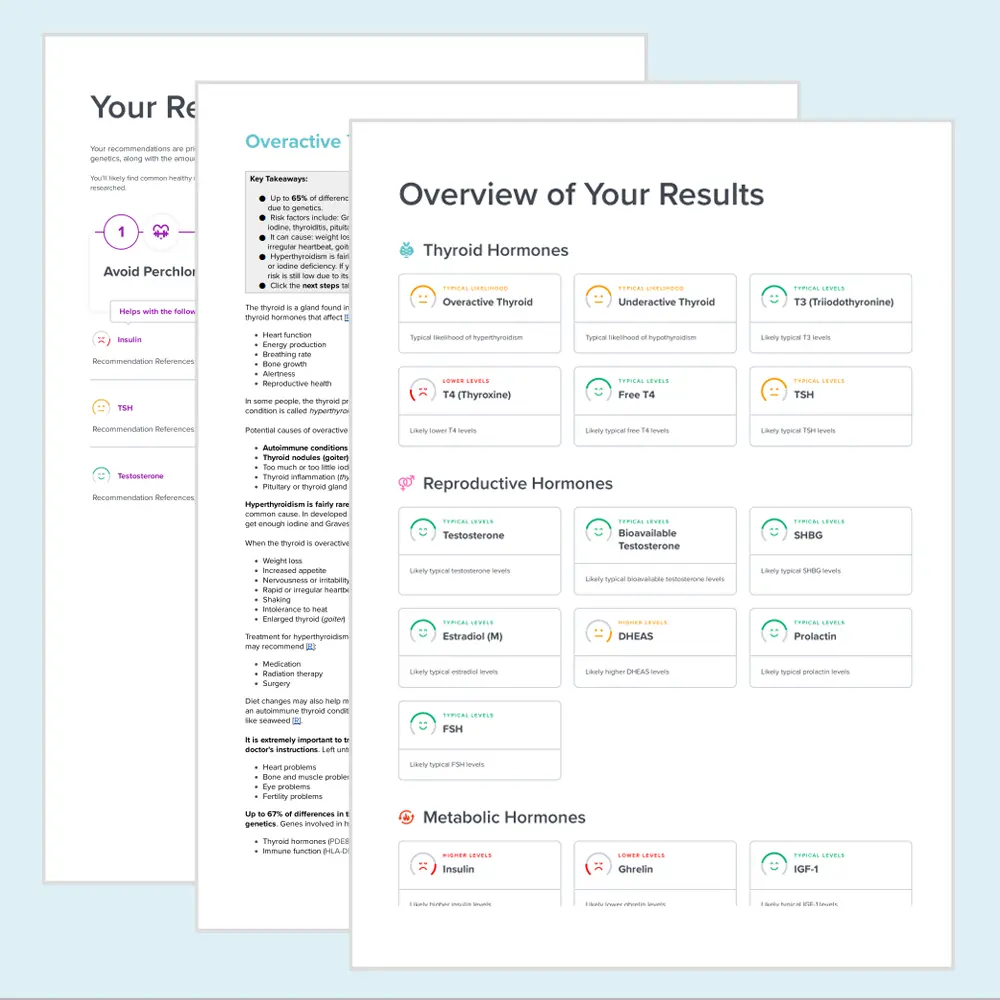
The DNA Hormone Health test from GetTested is a genetic analysis designed to illuminate your body's hormone health landscape. This comprehensive test examines specific genes linked to crucial hormones. These include T3 (Triiodothyronine), T4 (Thyroxine), Free T4, TSH (Thyroid-Stimulating Hormone), Testosterone, Bioavailable Testosterone, FSH (Follicle-Stimulating Hormone), and Ghrelin. It provides insights into how your body produces, regulates, and metabolizes these essential hormones. Understanding your genetic predispositions can help you tailor your lifestyle, diet, and possibly your supplementation. This customization will optimize your hormone health.
Why Choose This Test?
Optimizing hormone health is crucial for overall well-being, impacting energy levels, metabolism, mood, and more. Moreover, this DNA test is perfect for individuals seeking to understand their unique hormone health profile, those experiencing symptoms that could be hormone-related, or anyone interested in personalized health optimization.
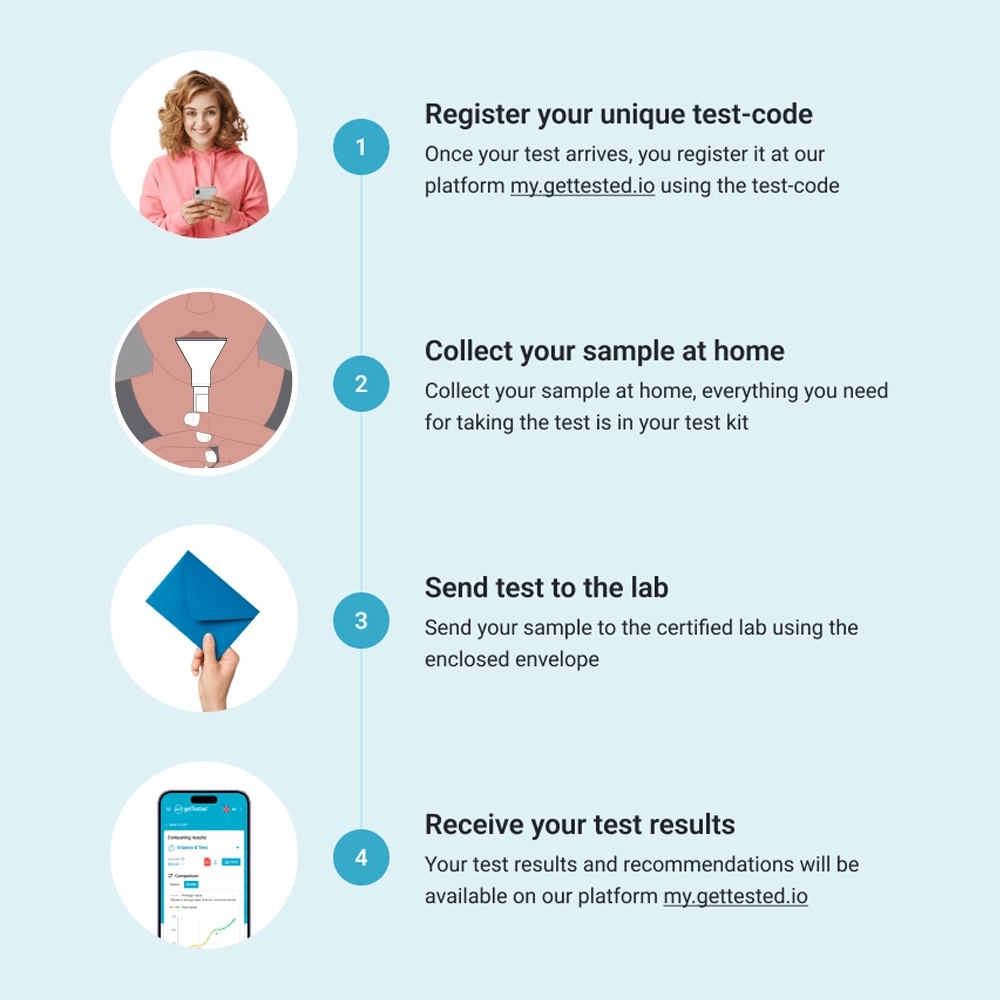
How It Works
- Firstly, order the Test: Have the DNA Hormone Health test kit sent directly to your home.
- Secondly, collect Your Sample: Follow our simple guide to collect your saliva sample.
- Thirdly, send It Back: Mail your sample to our lab using the prepaid envelope.
- Finally, discover Your Hormone Health: Receive a detailed report within 6-8 weeks, offering insights into your hormone health with actionable recommendations.
Privacy and Integrity
GetTested respects your privacy ardently. We destroy your DNA and sample after analysis, linking them only to your unique test ID. We ensure your results remain confidential, never shared with third parties, and provide you with the option to delete your results after review.
FAQ
How is the DNA Hormone Health test carried out?
How quickly will I receive my results?
When should I take the test?
Example Report
Example of DNA Hormone Health Test
Related Products
-
DNA Combo 3 - Order any 3 tests
You can combine any of our DNA tests to get a discount. Combine any 3 reports for £ ...£ 279,00 Add to cart -
Estrogen & progesterone test
GetTested's Estrogen & Progesterone Test measures the absolute levels and the rat...£ 79,00 Add to cart -
Menopause test 2-pack
Discover quickly if you are in menopause or perimenopause with the help of our menopa...£ 19,00 Add to cart -
Women's Hormone Test
Women's Hormone Test analyses cortisol, estrogen, progesterone, testosterone, DHEA, a...£ 159,00 Add to cart
You may also like…
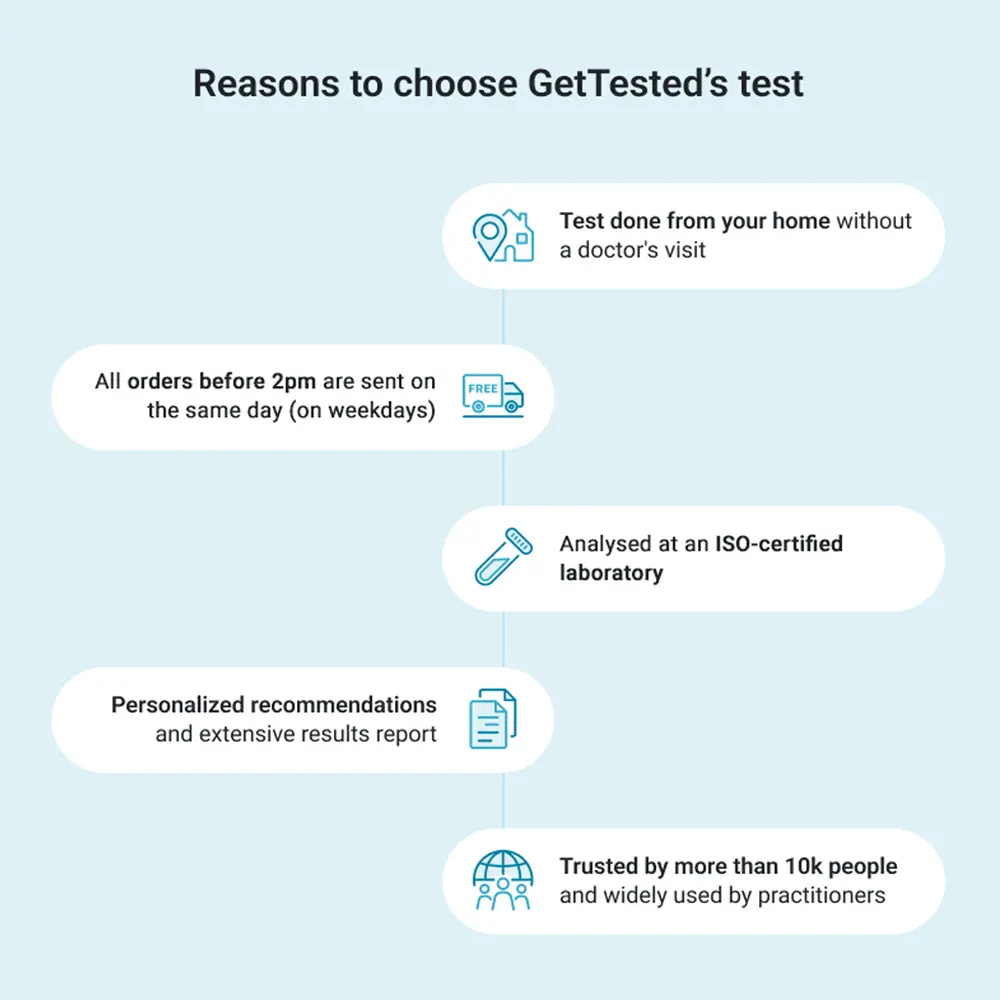
Trusted by over 10.000+ customers


“The home test was straightforward with easy to follow instructions. The test result was detailed and clear in its presentation. Also had the opport...”
Richard

“We looked at a lot of companies offering the same services but made the decision to go forward with get tested because the labs are based here in t...”
Natasha

“There are other providers out there, I have tried 3. Gettested was the fastest and the customer service was the best. I even received my test on th...”
Alan

“Absolutely perfect, results came after few days and finally after a lots of times visited GP, we finally know why my son has eczema - he is allergy...”
E

“I found them to be professional and not too long a wait for results. Helpful with any after questions. Will be using them again if need be. I highl...”
Eileen

“I really value my test and to see the results. It is a jungle when your health is online. So get accurate and precise knowledge is a gift, as it is...”










































































Leave a Reply